the lewis dot structure for sodium|Lewis Structure Finder : Tuguegarao A Lewis electron dot symbol (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of . The city of Philadelphia in Asia Minor was named after Attalos II, who was known for his loyalty to his brother Eumenes II. Philadelphia in the Book of Revelation. Centuries after the city of Philadelphia was founded, when the Christian writer John of Patmos was writing the Book of Revelation near the end of the first century AD, he .
the lewis dot structure for sodium,A step-by-step explanation of how to draw the Lewis dot structure for Na (Sodium). I show you where Sodium is on the periodic table and how to determine how many valence electrons it has. A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that .A Lewis electron dot symbol (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of .
the lewis dot structure for sodiumLewis dot structures represent atoms with their atomic symbol surrounded by valence electrons, which are represented as dots. This type of symbolic representation can .
This widget gets the Lewis structure of chemical compounds. Send feedback | Visit Wolfram|Alpha. Get the free "Lewis Structure Finder" widget for your website, blog, .Lewis Symbols. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol .
Thus, the Lewis dot formula for sodium is: Look at the electron configuration for magnesium. Magnesium is element #12. To draw the Lewis electron .
the lewis dot structure for sodium Lewis Structure Finder Lewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a molecule. These Lewis . Draw the Lewis dot structure of a given molecule or ion. Draw resonance structures of some molecules. Assign formal charge to an atom in a dot structure. .Summary: The Lewis dot diagram for sodium shows the arrangement of its valence electron. Sodium has one valence electron, which is represented by a single dot in the .
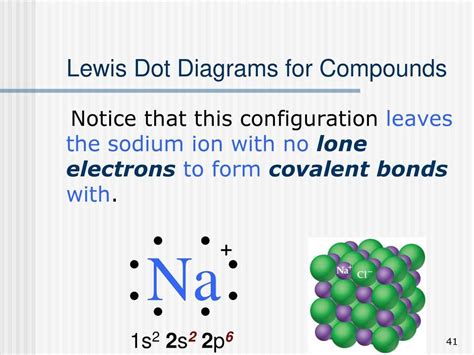
This would result in a negative charge for chlorine and a positive charge for sodium. Steps to draw Lewis dot structure of NaCl. Step 1: Count the available valence electrons. In the periodic table, sodium is in the first . This page titled 4.1: Lewis Electron Dot Structures is shared under a CK-12 license and was authored, remixed, and/or curated by CK-12 Foundation via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request. Ionic substances are completely held together by ionic bonds.A Lewis dot structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. . With the next element, sodium, the process starts over with a single electron because sodium has a single electron in its highest-numbered shell . Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Add an electron for every negative (-) charge, .A sodium atom has 11 electrons, but only one is a valence electron. The other 10 are inside a closed shell with a Neon electron configuration. . the Lewis-dot structure correctly predicts that there will be a triple bond between nitrogen atoms: This triple bond is very strong. The strength of the triple bond makes the N 2 molecule very stable .

A step-by-step explanation of how to draw the NaNO3 Lewis Dot Structure.For NaNO3 we have an ionic compound and we need to take that into account when we dra.
Q. (a) Draw electron dot structure of Sodium (Na), Oxygen (O) and Magnesium (Mg). (b) Why do ionic compounds have high melting points? Q. Draw the electron dot structure of a H 2 S molecule. [1 Mark] Q. Draw the electron dot diagram and structure of . (b) magnesium chloride . View More.Table 2.7.2 2.7. 2: Lewis Dot Symbols for the Elements in Period 2. Ionic compounds are produced when a metal bonds with a nonmetal. Stability is achieved for both atoms once the transfer of electrons has occurred. The image below shows how sodium and chlorine bond to form the compound sodium chloride.Lewis Structure Finder The strength of ionic bonding depends on the magnitude of the charges and the sizes of the ions. 10.3: Lewis Structures of Ionic Compounds- Electrons Transferred is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The tendency to form species that have eight electrons in the valence shell . Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a “skeleton structure.”.
A step-by-step explanation of how to draw the Na3P Lewis Dot Structure.For Na3P we have an ionic compound and we need to take that into account when we draw .The Lewis dot diagram is a visual representation of the valence electrons in an atom. It helps to understand the bonding and structure of molecules. In the case of sodium (Na), the Lewis dot diagram shows the arrangement of its valence electrons. For sodium, the atomic number is 11, and it has an electron configuration of 2-8-1. Sodium, a metal in Group 1, loses one electron to become a +1 ionIodine, a non-metal in Group 17, gains one electron to become a -1 ion.Together, they combin.Question #31400. Lewis structures (also known as Lewis dot diagrams, electron dot diagrams,"Lewis Dot formula" Lewis dot structures, and electron dot structures) are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any .

A step-by-step explanation of how to draw the NaCl Lewis Dot Structure (Sodium chloride).For NaCl we have an ionic compound and we need to take that into acc.
Table 4.5.2 4.5. 2: Lewis Dot Symbols for the Elements in Period 2. Ionic compounds are produced when a metal bonds with a nonmetal. Stability is achieved for both atoms once the transfer of electrons has occurred. The image below shows how sodium and chlorine bond to form the compound sodium chloride.
A step-by-step explanation of how to draw the NaCN Lewis Dot Structure.For NaCN we have an ionic compound and we need to take that into account when we draw .
We can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using Lewis dot symbols: The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with .
the lewis dot structure for sodium|Lewis Structure Finder
PH0 · The Sodium Lewis Dot Diagram: Understanding the Bonding
PH1 · Lewis Structure Finder
PH2 · Lewis Dot Symbols and Lewis Structures (Writing Lewis Symbols
PH3 · Lewis Dot Structures
PH4 · Lewis Dot Structure for Sodium (Na)
PH5 · High School Chemistry/Lewis Electron Dot Diagrams
PH6 · 9.2: Lewis Electron Dot Diagrams
PH7 · 7.3 Lewis Symbols and Structures
PH8 · 7.2 Lewis Dot Structures – Introduction to Chemistry
PH9 · 6.1 Lewis Electron Dot Symbols